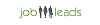Salario promedio: €3797 /mensual
Más estadísticasRecibir nuevas ofertas de empleo al correo
- ...CRA II - Sponsor Dedicated - Madrid/Barcelona, Spain. Min 2 years of experience as CRA. Updated: Yesterday Location: ESP-Remote (Madrid) Job ID: 25104990 Description CRA II - Sponsor Dedicated - Madrid/Barcelona, Spain. Min 2 years of experience as CRA....Ofertas de empleo recomendadasInterinoContratoRemotoInicio inmediato
- ...CRA II - Sponsor Dedicated - Madrid/Barcelona, Spain Updated: Yesterday Location: Madrid, Spain Job ID: 25104991 Description Syneos Health® is a leading fully integrated biopharmaceutical solutions organization built to accelerate customer success. We...Ofertas de empleo recomendadasInterinoContratoRemotoInicio inmediato
- ...Maintains knowledge of ICH/GCP guidelines, relevant regulations and SOPs; completes required training. For Real World Late Phase, the CRA II may use Site Management Associate II title with additional responsibilities including lifecycle site support, chart abstraction,...Ofertas de empleo recomendadasInterinoContratoRemotoTrabajo híbrido
- A leading biopharmaceutical solutions organization in Madrid/Barcelona is looking for a Clinical Research Associate II to oversee clinical research studies. This role involves performing site qualifications, managing site activities, and ensuring compliance with regulatory...Ofertas de empleo recomendadasRemoto
- A global contract research organization is seeking a Clinical Research Associate (CRA) for a home-based position in Sevilla. The role involves monitoring clinical studies in phases II-III, ensuring compliance with Good Clinical Practices, and working closely with investigators...Ofertas de empleo recomendadasContratoDesde casa
- A leading clinical development organization is seeking a Clinical Research Associate II for its operations in Madrid/Barcelona, Spain. The ideal candidate should have a Bachelor's degree or relevant field experience, along with at least 2 years in monitoring. This role...Ofertas de empleo recomendadasRemoto
- A leading biopharmaceutical solutions organization is looking for a Clinical Research Associate II in Barcelona. This role involves performing site qualifications, monitoring, and ensuring compliance with regulatory requirements. Candidates should have a Bachelor’s degree...Ofertas de empleo recomendadasRemoto
- A biopharmaceutical solutions organization is seeking a Clinical Research Associate II (CRA II) dedicated to monitoring clinical studies in Spain. The role involves ensuring compliance with regulations, managing trial sites, and supporting patient recruitment. The ideal...Ofertas de empleo recomendadasRemoto
- A leading biopharmaceutical solutions organization is seeking a CRA II to oversee clinical research sites in Barcelona. The role includes managing site compliance, conducting monitoring visits, and ensuring data integrity throughout clinical trials. Candidates should have...Ofertas de empleo recomendadas
- A leading clinical research organization in Madrid is looking for a Clinical Research Associate (CRA) to monitor clinical studies in phases II-III. The ideal candidate should have at least one year of monitoring experience, a Bachelor's degree in life-science, and be fluent...Ofertas de empleo recomendadasTiempo completo
- A leading biopharmaceutical solutions organization is seeking a Clinical Research Associate to oversee clinical research studies, ensuring compliance with regulations. The role requires a Bachelor's degree or RN and at least 1 year of oncology experience, combined with ...Ofertas de empleo recomendadasRemoto
- As a CRA sponsor dedicated, you will be joining the world’s largest & most comprehensive clinical research organisation, powered by healthcare... ...What you will be doing: • You will monitor multiple Phase I, II, III & IV clinical trial sites, across different therapeutic...Ofertas de empleo recomendadasAutónomoEmpleo permanenteRemoto
- ...Work Schedule Environmental Conditions Job Description Join Us as a Clinical Research Associate (Level II) Make an Impact at the Forefront of Innovation. You will be fully dedicated to one of our clients. As part of our global team youll have the opportunity...Ofertas de empleo recomendadasTiempo completoTrabajar en la oficinaDesde casaRemotoTurno de noche
- ...Spain. Job Overview Monitoring clinical studies in phases II-III Assuring adherence to Good Clinical Practices, investigator... ...Bachelor degree in life-science Min 1 year monitoring experience as CRA within a CRO, Pharma, Biotech Experience in commercial studies...Ofertas de empleo recomendadas
- ...clinical development, peri-approval & market access. They are currently seeking a CRA sponsor dedicated home based in Andalusia. Job Overview Monitoring clinical studies in phases II-III Assuring adherence to Good Clinical Practices, investigator integrity, and...Ofertas de empleo recomendadasDesde casa
- A global CRO in Barcelona is seeking a Clinical Research Associate (CRA) for monitoring clinical studies in phases II-III. This role requires a Bachelor degree in life-science, at least one year of monitoring experience, and fluency in both English and Spanish. The ideal...
- ...Phase studies in Spain. You will monitor clinical studies in phases II-III, ensure adherence to Good Clinical Practices, and supervise... ...have a Bachelor degree in life-science, at least 1 year of CRA experience, and fluency in English and Spanish. Excellent communication...
- ...development, peri-approval & market access. They are currently seeking a CRA / Clinical Research Associate Regional Monitoring in Madrid Job Overview Monitoring clinical studies in phases II-III Assuring adherence to Good Clinical Practices, investigator integrity...
- ...presencia nacional e internacional un/a Clinical Research Associate (CRA) para incorporarse a su equipo en Barcelona. Se trata de una posición orientada a la monitorización de ensayos clínicos en fases II-III. Responsibilities Monitorización de centros hospitalarios...Desde casaTrabajo híbrido
- ...CRA I in Valencia with experience as CRA. Sponsor dedicated. Updated: December 19, 2025 Location: ESP-Remote (Barcelona) Job ID: 25104668 Description CRA I in Valencia with experience as CRA. Sponsor dedicated. Syneos Health® is a leading fully integrated...InterinoContratoRemotoInicio inmediato
- CRA II ICON plc is a world-leading healthcare intelligence and clinical research organization. We’re proud to foster an inclusive environment driving innovation and excellence, and we welcome you to join us on our mission to shape the future of clinical development....AutónomoEmpleo permanente
- Una empresa de tecnología de seguridad busca un Operador/a de Central Receptora de Alarmas en Madrid. Las responsabilidades incluyen gestionar alertas de seguridad y activar protocolos. Se requiere experiencia previa en atención al cliente, con disponibilidad para turnos...Turno rotativo
- .Operador/a de Central Receptora de Alarmas (CRA) - Temporal page is loaded## Operador/a de Central Receptora de Alarmas (CRA) - Temporallocations: Las Rozas-Madrid-Spaintime type: Full timeposted on: Posted Todayjob requisition id: WD30260577# **Operador/a de Central Receptora...TemporalTurno rotativoTurno de mañanaTurno de tardeTurno de nocheTrabajo por turnos
- A leading clinical research organization based in Barcelona seeks a motivated Sr CRA to lead site monitoring for clinical trials. The ideal candidate will have at least 2 years of clinical monitoring experience and expertise in managing trial-related issues. Responsibilities...
- Uma empresa do setor de bioprodutos procura um profissional para atuar em laboratório, realizando ensaios e garantindo a qualidade e segurança dos processos. O candidato ideal deve ter curso técnico em Química ou Celulose e Papel, além de perfil analítico e iniciativa. ...
- A global health services organization is seeking a Senior Clinical Research Associate (Sr CRA) in Barcelona. The Sr CRA will be responsible for conducting site monitoring for clinical trials, ensuring protocol compliance, and providing leadership to the CRA team. Ideal...
- Se busca personal de seguridad para un servicio importante en Zamudio (Bizkaia), dentro del Parque Tecnológico. Las responsabilidades principales incluirán el control de accesos, asegurando que se cumplan las normativas de entrada y salida de forma rigurosa pero amable...TemporalTiempo completoCon coche propioTurno rotativoTurno de mañanaTurno de tardeTrabajo por turnos
- A leading healthcare organization in Barcelona is seeking an Associate Outcomes Researcher to join their team. The ideal candidate will hold a PhD in Life Sciences and have at least 4 years of experience in a professional environment. Responsibilities include leading literature...
- A leading healthcare intelligence organization is seeking a Senior Clinical Trial Assistant to support clinical trials in Madrid. This role involves extensive administrative tasks, managing documentation, and facilitating effective communication among stakeholders. Candidates...Trabajar en la oficina
- Clinical Trial Assistant- Sponsor Dedicated based in Madrid page is loaded## Clinical Trial Assistant- Sponsor Dedicated based in Madridlocations: Madrid, Spaintime type: Full timeposted on: Posted Todayjob requisition id: R1488999**Job Overview** Perform daily administrative...Inicio inmediato